BFE1224 (Fosravuconazole)
-
- Fosravuconazole (tri-azole
antifungal agent discovered by
Eisai ) has been improved as far better compound in
solubility and bioavailability by dihydrogen phosphonoxy
methoxylated Ravuconazole.
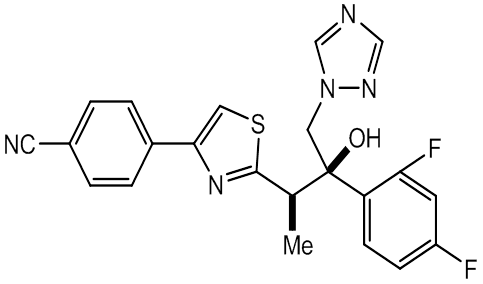
Ravuconazole
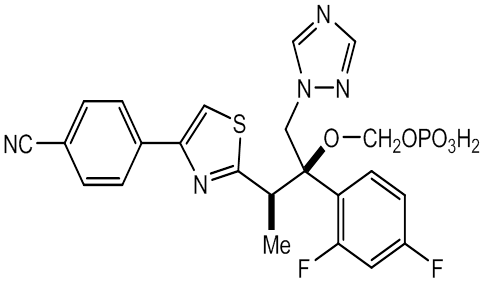
Fosravuconazole
What’s Onychomycosis?
Onychomycosis is mycosis develops on nail surface and /or nail bed 10% of population are affected with onycomycosis. Risk factors for the disease are tinea pedis, nail-lipodystrophy, advanced age, male, cardiovascular disease. Infection on foot nails are 10 times many than hand nails. 60 to 80% of the cause of cases are dermatophytes. Candidal nail tinea in some cases affects immunodeficient patients and chronic mucocutaneous.(Referred from Merck Manual 18 version in Japanese(2006)
-
Therapeutic Field
Onychomycosis(e.g. nail tinea, nail candidiasis )
Medical Needs
Medical treatment of onychomycosis has been done mainly by oral antifungal drugs, but relapse cases are many and that is one of the reasons of few complete cure. There is a high medical need due to better profiles of fosravuconazole than existing drugs.
Marketability
- Population of onychomycosis; in Japan 12 million patients and 10% of whole population of the nation (potentially huge market )
- Seven biggest market of this disease : US, Japan, Europe (UK, Germany, France, Italy, Spain)
Significant Competitive Advantages
- Safety profile is better than two existing drugs.
- Administration compliance is improved.
- Long duration of treatment efficacy (long t1/2) could be also effective in prevention of recurrence and relapse of the disease.
- Wide spectrum for causal fungi and higher MIC
Competing Products
- At present on the market there are two oral drugs and their generics which have indication of treatment of onychomycosis.
- No competitive oral drugs at clinical stages world wide. -
Development -
Development
Therapeutic
License
Indication
Development
Code No. Category Territory
BFE1224 antifunfal Japan onychomycosis approved
(oral) North America
Europe -
Published
report
with regard to POC (Proof of Concept) of Ravuconazole for
onychomycosis
Gupta AK, Leonardi C, Stoltz RR, Pierce PF, Conetta B; Ravuconazole onychomycosis group,A phase I/II randomized, double-blind, placebo-controlled, dose-ranging study evaluating the efficacy, safety and pharmacokinetics of ravuconazole in the treatment of onychomycosis,J Eur Acad Dermatol Venereol. 2005 Jul;19(4):437-43.